PTC Therapeutics, Inc. (PTCT)
Price:
68.98 USD
( - -1.52 USD)
Your position:
0 USD
ACTION PANEL
ABOUT
Check the
KEY TAKEAWAYS
ASK OUR AI ABOUT THE COMPANY (REGISTER FOR EARLY ACCESS)

(REGISTER FOR EARLY ACCESS) CHOOSE A PROMPT ABOVE TO ASK OUR AI ABOUT THE SPECIFIC INFORMATION
SIMILAR COMPANIES STI SCORE
Similar STI Score
Incyte Corporation
VALUE SCORE:
10
2nd position
ADMA Biologics, Inc.
VALUE SCORE:
11
The best
Harmony Biosciences Holdings, Inc.
VALUE SCORE:
12
FUNDAMENTALS
FUNDAMENTALS
FUNDAMENTALS
FUNDAMENTALS PER SHARE
TECHNICAL
DIVIDEND
SIMILAR COMPANIES
DESCRIPTION
PTC Therapeutics, Inc., a biopharmaceutical company, focuses on the discovery, development, and commercialization of medicines to patients with rare disorders. Its portfolio pipeline includes commercial products and product candidates in various stages of development, including clinical, pre-clinical and research and discovery stages, focuses on the development of treatments for multiple therapeutic areas, such as rare diseases. The company offers Translarna and Emflaza for the treatment of Duchenne muscular dystrophy in the European Economic Area and the United States, as well as to treat nonsense mutation Duchenne muscular dystrophy in Brazil and Russia; commercializes Tegsedi and Waylivra for the treatment of rare diseases in Latin America and the Caribbean; and markets Evrysdi for the treatment of spinal muscular atrophy in adults and children two months and older in Brazil. The company's splicing platform includes PTC518, which is being developed for the treatment of Huntington's disease. PTC Therapeutics, Inc. has collaborations with F. Hoffman-La Roche Ltd and Hoffman-La Roche Inc., as well as the Spinal Muscular Atrophy Foundation to advance drug discovery and development research in regenerative medicine; and Akcea Therapeutics, Inc. to commercialize Tegsedi and Waylivra for the treatment of rare diseases in Latin America and the Caribbean. PTC Therapeutics, Inc. was incorporated in 1998 and is headquartered in South Plainfield, New Jersey.
NEWS

PTC Therapeutics to Participate at Upcoming Investor Conferences
prnewswire.com
2026-02-23 08:00:00WARREN, N.J., Feb. 23, 2026 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that its executives will speak at the following conferences: TD Cowen 46th Annual Health Care Conference 2026 Monday, March 2 at 9:50 a.m.

PTC Therapeutics: Sephience As A Major Growth Driver
seekingalpha.com
2026-02-23 07:26:20PTC Therapeutics is transitioning its growth engine from legacy drugs to new launches like Sephience, targeting rare genetic diseases with high unmet need. Pipeline catalysts, notably Votoplam for Huntington's disease and Vatiquinone for Friedreich's ataxia, offer significant upside but carry clinical trial risks. PTCT's competitive edge lies in its oral small molecule platform, collaborative development model, and strong pricing power in ultra-rare disease markets.

PTC Therapeutics (NASDAQ:PTCT) CAO Sells $172,983.84 in Stock
defenseworld.net
2026-02-23 06:24:46PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) CAO Christine Marie Utter sold 2,494 shares of the stock in a transaction that occurred on Wednesday, February 18th. The shares were sold at an average price of $69.36, for a total value of $172,983.84. Following the completion of the sale, the chief accounting officer directly owned

Insider Selling: PTC Therapeutics (NASDAQ:PTCT) EVP Sells $172,290.24 in Stock
defenseworld.net
2026-02-23 06:24:46PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) EVP Lee Scott Golden sold 2,484 shares of the company's stock in a transaction that occurred on Wednesday, February 18th. The stock was sold at an average price of $69.36, for a total value of $172,290.24. Following the transaction, the executive vice president directly owned 89,944 shares

Matthew Klein Sells 7,371 Shares of PTC Therapeutics (NASDAQ:PTCT) Stock
defenseworld.net
2026-02-22 05:37:02PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) CEO Matthew Klein sold 7,371 shares of PTC Therapeutics stock in a transaction that occurred on Wednesday, February 18th. The stock was sold at an average price of $69.36, for a total value of $511,252.56. Following the transaction, the chief executive officer owned 387,082 shares in the

19,142 Shares in PTC Therapeutics, Inc. $PTCT Acquired by Brummer Multi Strategy AB
defenseworld.net
2026-02-22 04:58:44Brummer Multi Strategy AB purchased a new stake in shares of PTC Therapeutics, Inc. (NASDAQ: PTCT) during the third quarter, according to the company in its most recent 13F filing with the SEC. The institutional investor purchased 19,142 shares of the biopharmaceutical company's stock, valued at approximately $1,175,000. PTC Therapeutics makes up 21.4%

PTC Therapeutics, Inc. (PTCT) Q4 2025 Earnings Call Transcript
seekingalpha.com
2026-02-20 13:24:59PTC Therapeutics, Inc. (PTCT) Q4 2025 Earnings Call Transcript

PTC Therapeutics (PTCT) Q4 Earnings: Taking a Look at Key Metrics Versus Estimates
zacks.com
2026-02-19 20:31:12The headline numbers for PTC Therapeutics (PTCT) give insight into how the company performed in the quarter ended December 2025, but it may be worthwhile to compare some of its key metrics to Wall Street estimates and the year-ago actuals.

PTC Therapeutics (PTCT) Reports Q4 Loss, Misses Revenue Estimates
zacks.com
2026-02-19 20:01:10PTC Therapeutics (PTCT) came out with a quarterly loss of $1.67 per share versus the Zacks Consensus Estimate of a loss of $0.21. This compares to a loss of $0.24 per share a year ago.

PTC Therapeutics Provides Corporate Update and Reports Fourth Quarter and Full Year 2025 Financial Results
prnewswire.com
2026-02-19 16:01:00– Full-year 2025 product and royalty revenue of $831M, exceeding guidance – – Strong Sephience™ (sepiapterin) uptake since 2H 2025 launch with fourth quarter and 2025 revenue of $92M and $111M, respectively – – Cash of $1.95B as of December 31, 2025 – WARREN, N.J., Feb. 19, 2026 /PRNewswire/ -- PTC Therapeutics, Inc., (NASDAQ: PTCT) today announced a corporate update and financial results for the fourth quarter and full year ending December 31, 2025.

Ahead of PTC Therapeutics (PTCT) Q4 Earnings: Get Ready With Wall Street Estimates for Key Metrics
zacks.com
2026-02-16 10:15:47Besides Wall Street's top-and-bottom-line estimates for PTC Therapeutics (PTCT), review projections for some of its key metrics to gain a deeper understanding of how the company might have fared during the quarter ended December 2025.

PTC Therapeutics Provides Regulatory Update on Translarna™
prnewswire.com
2026-02-12 17:00:00WARREN, N.J., Feb. 12, 2026 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) announced today that it has withdrawn the New Drug Application (NDA) resubmission for Translarna™ (ataluren) for the treatment of nonsense mutation Duchenne muscular dystrophy (DMD) following U.S. Food and Drug Administration (FDA) feedback on the application review.

PTC Therapeutics (PTCT) Expected to Beat Earnings Estimates: What to Know Ahead of Q4 Release
zacks.com
2026-02-12 11:06:03PTC Therapeutics (PTCT) possesses the right combination of the two key ingredients for a likely earnings beat in its upcoming report. Get prepared with the key expectations.

PTC Therapeutics to Report Fourth Quarter and Full Year 2025 Financial Results on Thursday, Feb. 19, 2026
prnewswire.com
2026-02-05 08:00:00WARREN, N.J., Feb. 5, 2026 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) announced today that the company will host a webcast conference call to report its fourth quarter and full year 2025 financial results and provide an update on the company's business and outlook on Thursday, Feb. 19, 2026, at 4:30 p.m.
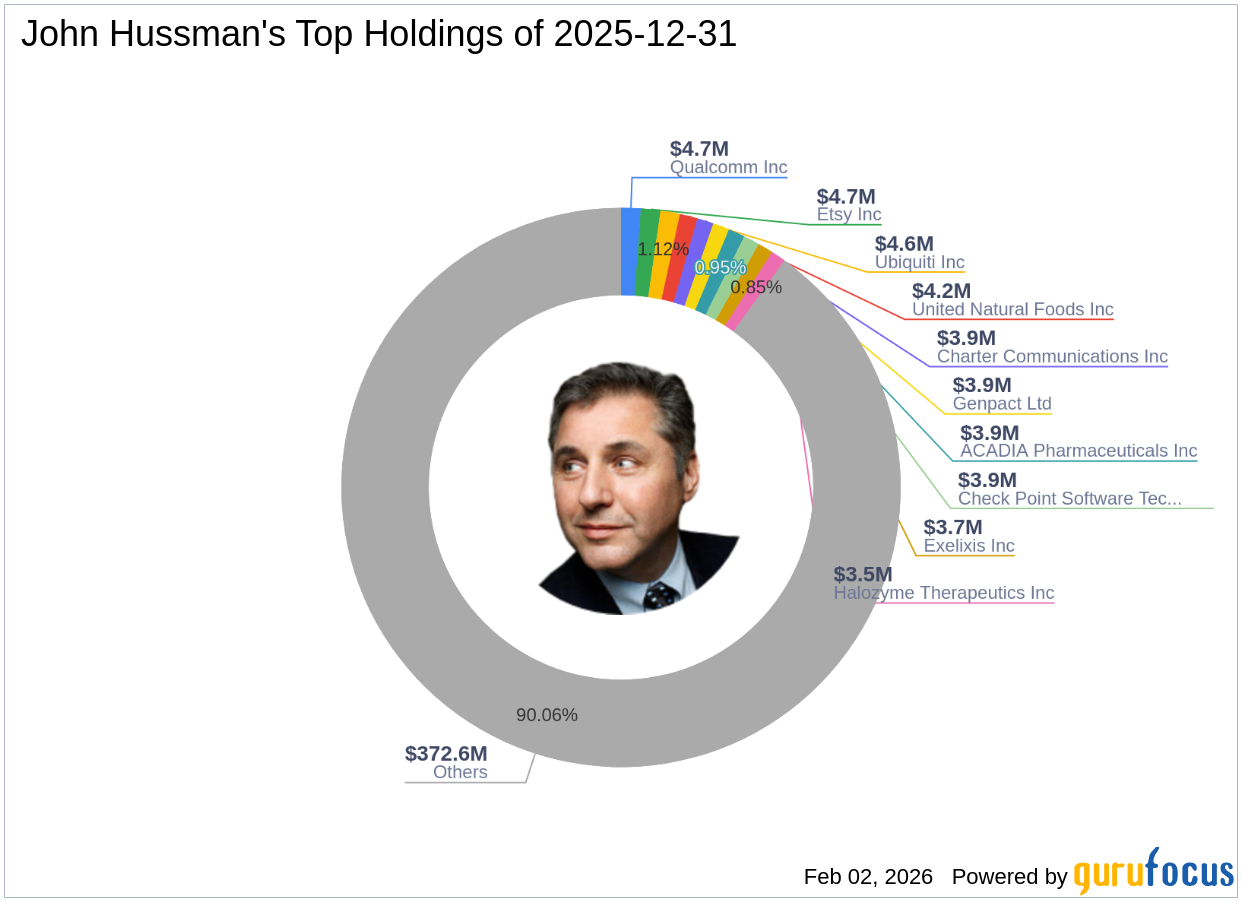
John Hussman's Strategic Moves: Qualcomm Inc. Takes Center Stage with 1.13% Portfolio Impact
gurufocus.com
2026-02-02 13:01:00Exploring the Investment Strategies of John Hussman (Trades, Portfolio) in Q4 2025 John Hussman (Trades, Portfolio) recently submitted the 13F filing for the f

PTC Therapeutics, Inc. (NASDAQ:PTCT) Given Average Rating of “Moderate Buy” by Analysts
defenseworld.net
2026-02-02 01:43:10PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) has earned a consensus rating of "Moderate Buy" from the sixteen ratings firms that are covering the company, MarketBeat Ratings reports. One analyst has rated the stock with a sell rating, five have issued a hold rating and ten have assigned a buy rating to the company.
No data to display

PTC Therapeutics to Participate at Upcoming Investor Conferences
prnewswire.com
2026-02-23 08:00:00WARREN, N.J., Feb. 23, 2026 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that its executives will speak at the following conferences: TD Cowen 46th Annual Health Care Conference 2026 Monday, March 2 at 9:50 a.m.

PTC Therapeutics: Sephience As A Major Growth Driver
seekingalpha.com
2026-02-23 07:26:20PTC Therapeutics is transitioning its growth engine from legacy drugs to new launches like Sephience, targeting rare genetic diseases with high unmet need. Pipeline catalysts, notably Votoplam for Huntington's disease and Vatiquinone for Friedreich's ataxia, offer significant upside but carry clinical trial risks. PTCT's competitive edge lies in its oral small molecule platform, collaborative development model, and strong pricing power in ultra-rare disease markets.

PTC Therapeutics (NASDAQ:PTCT) CAO Sells $172,983.84 in Stock
defenseworld.net
2026-02-23 06:24:46PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) CAO Christine Marie Utter sold 2,494 shares of the stock in a transaction that occurred on Wednesday, February 18th. The shares were sold at an average price of $69.36, for a total value of $172,983.84. Following the completion of the sale, the chief accounting officer directly owned

Insider Selling: PTC Therapeutics (NASDAQ:PTCT) EVP Sells $172,290.24 in Stock
defenseworld.net
2026-02-23 06:24:46PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) EVP Lee Scott Golden sold 2,484 shares of the company's stock in a transaction that occurred on Wednesday, February 18th. The stock was sold at an average price of $69.36, for a total value of $172,290.24. Following the transaction, the executive vice president directly owned 89,944 shares

Matthew Klein Sells 7,371 Shares of PTC Therapeutics (NASDAQ:PTCT) Stock
defenseworld.net
2026-02-22 05:37:02PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) CEO Matthew Klein sold 7,371 shares of PTC Therapeutics stock in a transaction that occurred on Wednesday, February 18th. The stock was sold at an average price of $69.36, for a total value of $511,252.56. Following the transaction, the chief executive officer owned 387,082 shares in the

19,142 Shares in PTC Therapeutics, Inc. $PTCT Acquired by Brummer Multi Strategy AB
defenseworld.net
2026-02-22 04:58:44Brummer Multi Strategy AB purchased a new stake in shares of PTC Therapeutics, Inc. (NASDAQ: PTCT) during the third quarter, according to the company in its most recent 13F filing with the SEC. The institutional investor purchased 19,142 shares of the biopharmaceutical company's stock, valued at approximately $1,175,000. PTC Therapeutics makes up 21.4%

PTC Therapeutics, Inc. (PTCT) Q4 2025 Earnings Call Transcript
seekingalpha.com
2026-02-20 13:24:59PTC Therapeutics, Inc. (PTCT) Q4 2025 Earnings Call Transcript

PTC Therapeutics (PTCT) Q4 Earnings: Taking a Look at Key Metrics Versus Estimates
zacks.com
2026-02-19 20:31:12The headline numbers for PTC Therapeutics (PTCT) give insight into how the company performed in the quarter ended December 2025, but it may be worthwhile to compare some of its key metrics to Wall Street estimates and the year-ago actuals.

PTC Therapeutics (PTCT) Reports Q4 Loss, Misses Revenue Estimates
zacks.com
2026-02-19 20:01:10PTC Therapeutics (PTCT) came out with a quarterly loss of $1.67 per share versus the Zacks Consensus Estimate of a loss of $0.21. This compares to a loss of $0.24 per share a year ago.

PTC Therapeutics Provides Corporate Update and Reports Fourth Quarter and Full Year 2025 Financial Results
prnewswire.com
2026-02-19 16:01:00– Full-year 2025 product and royalty revenue of $831M, exceeding guidance – – Strong Sephience™ (sepiapterin) uptake since 2H 2025 launch with fourth quarter and 2025 revenue of $92M and $111M, respectively – – Cash of $1.95B as of December 31, 2025 – WARREN, N.J., Feb. 19, 2026 /PRNewswire/ -- PTC Therapeutics, Inc., (NASDAQ: PTCT) today announced a corporate update and financial results for the fourth quarter and full year ending December 31, 2025.

Ahead of PTC Therapeutics (PTCT) Q4 Earnings: Get Ready With Wall Street Estimates for Key Metrics
zacks.com
2026-02-16 10:15:47Besides Wall Street's top-and-bottom-line estimates for PTC Therapeutics (PTCT), review projections for some of its key metrics to gain a deeper understanding of how the company might have fared during the quarter ended December 2025.

PTC Therapeutics Provides Regulatory Update on Translarna™
prnewswire.com
2026-02-12 17:00:00WARREN, N.J., Feb. 12, 2026 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) announced today that it has withdrawn the New Drug Application (NDA) resubmission for Translarna™ (ataluren) for the treatment of nonsense mutation Duchenne muscular dystrophy (DMD) following U.S. Food and Drug Administration (FDA) feedback on the application review.

PTC Therapeutics (PTCT) Expected to Beat Earnings Estimates: What to Know Ahead of Q4 Release
zacks.com
2026-02-12 11:06:03PTC Therapeutics (PTCT) possesses the right combination of the two key ingredients for a likely earnings beat in its upcoming report. Get prepared with the key expectations.

PTC Therapeutics to Report Fourth Quarter and Full Year 2025 Financial Results on Thursday, Feb. 19, 2026
prnewswire.com
2026-02-05 08:00:00WARREN, N.J., Feb. 5, 2026 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) announced today that the company will host a webcast conference call to report its fourth quarter and full year 2025 financial results and provide an update on the company's business and outlook on Thursday, Feb. 19, 2026, at 4:30 p.m.

John Hussman's Strategic Moves: Qualcomm Inc. Takes Center Stage with 1.13% Portfolio Impact
gurufocus.com
2026-02-02 13:01:00Exploring the Investment Strategies of John Hussman (Trades, Portfolio) in Q4 2025 John Hussman (Trades, Portfolio) recently submitted the 13F filing for the f

PTC Therapeutics, Inc. (NASDAQ:PTCT) Given Average Rating of “Moderate Buy” by Analysts
defenseworld.net
2026-02-02 01:43:10PTC Therapeutics, Inc. (NASDAQ: PTCT - Get Free Report) has earned a consensus rating of "Moderate Buy" from the sixteen ratings firms that are covering the company, MarketBeat Ratings reports. One analyst has rated the stock with a sell rating, five have issued a hold rating and ten have assigned a buy rating to the company.










