PureTech Health plc (PRTC)
Price:
16.77 USD
( + 0.12 USD)
Your position:
0 USD
ACTION PANEL
ABOUT
Check the
KEY TAKEAWAYS
ASK OUR AI ABOUT THE COMPANY (REGISTER FOR EARLY ACCESS)

(REGISTER FOR EARLY ACCESS) CHOOSE A PROMPT ABOVE TO ASK OUR AI ABOUT THE SPECIFIC INFORMATION
SIMILAR COMPANIES STI SCORE
Similar STI Score
argenx SE
VALUE SCORE:
9
2nd position
ADMA Biologics, Inc.
VALUE SCORE:
11
The best
Aurinia Pharmaceuticals Inc.
VALUE SCORE:
12
FUNDAMENTALS
FUNDAMENTALS
FUNDAMENTALS
FUNDAMENTALS PER SHARE
TECHNICAL
DIVIDEND
SIMILAR COMPANIES
DESCRIPTION
PureTech Health plc, a clinical-stage biotherapeutics company, discovers, develops, and commercializes medicines for inflammatory, fibrotic and immunological, intractable cancers, lymphatic and gastrointestinal, neurological and neuropsychological, and other diseases in the United States. The company offers KarXT targeting muscarinic acetylcholine receptors to treat schizophrenia and psychosis in Alzheimer's disease; a regenerative biology platform for androgenetic alopecia, epithelial ageing, and other medical conditions; an immunomodulation platform to treat chronic and acute inflammatory disorders; oral therapies based on defined consortia of bacteria is isolated from the human microbiome; and therapies to treat cognitive dysfunction associated with depression, multiple sclerosis, post COVID and ICU, and cancer related conditions. It also provides hematopoietic stem cell based therapies for patients with blood cancer; a voice-based technology platform to detect voice changes linked to health conditions; and a technology platform for the oral delivery of biologics, vaccines, and other drugs. In addition, the company is developing LYT-100 to treat lymphedema, and other lymphatic flow disorders; LYT-200, a IgG4 monoclonal antibody to target galectin-9; LYT-210 to treat solid tumors; Glyph, a synthetic lymphatic targeting chemistry platform; Orasome technology to enable the oral administration of macromolecule therapeutic payloads; meningeal lymphatics platform to treat Alzheimer's and Parkinson's diseases; and Alivio technology platform for inflammation-targeted disease immunomodulation. PureTech Health plc has collaboration and license agreements with Boehringer Ingelheim International GMBH; Eli Lilly and Company; Imbrium Therapeutics L.P.; and Shionogi & Co., Ltd. The company was incorporated in 2015 and is headquartered in Boston, Massachusetts.
NEWS

PureTech to Present at the Leerink Partners Global Healthcare Conference
businesswire.com
2026-02-25 02:00:00BOSTON--(BUSINESS WIRE)--PureTech to Present at the Leerink Partners Global Healthcare Conference.

PureTech Announces Orphan Drug Designations Granted by the U.S. Food and Drug Administration and European Commission for Deupirfenidone (LYT-100) in Idiopathic Pulmonary Fibrosis
businesswire.com
2026-02-19 12:36:00BOSTON--(BUSINESS WIRE)--PureTech Announces Orphan Drug Designations Granted by the U.S. Food and Drug Administration and European Commission for Deupirfenidone in IPF.

PureTech Health plc (PRTC) Presents at 44th Annual J.P. Morgan Healthcare Conference Transcript
seekingalpha.com
2026-01-14 23:35:58PureTech Health plc (PRTC) Presents at 44th Annual J.P. Morgan Healthcare Conference Transcript

PureTech to Present at 44th Annual J.P. Morgan Healthcare Conference
businesswire.com
2026-01-06 07:00:00BOSTON--(BUSINESS WIRE)--PureTech to Present at 44th Annual J.P. Morgan Healthcare Conference.

PureTech Appoints Robert Lyne as Chief Executive Officer
businesswire.com
2025-12-18 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) ("PureTech" or the "Company"), a hub-and-spoke biotherapeutics company dedicated to giving life to science and transforming innovation into value, today announces that the Board of Directors has appointed Robert Lyne as Chief Executive Officer (CEO), and as a member of the Board of Directors, effective immediately. Robert Lyne commented: “I'm honored to lead PureTech as CEO at such an important moment in its evolution. Over.

Puretech gains after FDA meeting post-Phase II trial
proactiveinvestors.co.uk
2025-12-08 06:25:14PureTech Health (LSE:PRTC, NASDAQ:PRTC, OTC:PTCHF) shares rose 4.9% to 128p on Wednesday after the company said it had completed a successful end-of-Phase 2 meeting with the US Food and Drug Administration for deupirfenidone, its lead idiopathic pulmonary fibrosis candidate. The regulator's feedback supports advancement into a pivotal Phase 3 trial and confirms the suitability of the streamlined 505(b)(2) regulatory pathway, reducing a major point of uncertainty around the programme.

Cavendish sees Poolbeg’s POLB 001 gaining strategic importance as CRS research expands
proactiveinvestors.com
2025-12-08 06:15:00Cavendish has taken an upbeat view of Poolbeg Pharma PLC's (AIM:POLB, OTC:POLBF) prospects after the company’s anti-inflammatory candidate POLB 001 was...

Greengage plans AQSE float with bold Bitcoin-backed lending strategy
proactiveinvestors.com
2025-12-08 06:10:00Fintech Greengage has set out plans to list on the Access segment of the Aquis Growth Market... and it is doing so with an unusually ambitious twist....

Puretech gains after FDA meeting post-Phase II trial
proactiveinvestors.com
2025-12-08 05:54:00PureTech Health (LSE:PRTC, NASDAQ:PRTC, OTC:PTCHF) shares rose 4.9% to 128p on Wednesday after the company said it had completed a successful end-of-Phase 2...

Coinsilium notes Greengage’s plan to float on Aquis
proactiveinvestors.com
2025-12-08 04:27:00Coinsilium Group Limited (AQSE:COIN, OTCQB:CINGF) has welcomed news that Greengage, one of its portfolio companies, intends to list on the Aquis Stock...

PureTech Announces Successful End-of-Phase 2 Meeting with FDA for Deupirfenidone (LYT-100) in Idiopathic Pulmonary Fibrosis
businesswire.com
2025-12-08 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Announces Successful End-of-Phase 2 Meeting with FDA for Deupirfenidone (LYT-100) in Idiopathic Pulmonary Fibrosis.

PureTech's Founded Entity Gallop Oncology Announces Positive Initial Topline Data from Phase 1b Trial of LYT-200 in Relapsed/Refractory Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome
businesswire.com
2025-12-05 13:00:00BOSTON--(BUSINESS WIRE)--PureTech's Founded Entity Gallop Oncology Announces Positive Initial Topline Data for LYT-200 in Relapsed/Refractory Acute Myeloid Leukemia.

PureTech Presents New Phase 2b Analyses Demonstrating Consistent Safety and Efficacy of Deupirfenidone in Older Patients with Idiopathic Pulmonary Fibrosis (IPF), a Historically Undertreated Group
businesswire.com
2025-10-22 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Presents New Phase 2b Analyses Demonstrating Consistent Safety and Efficacy of Deupirfenidone in Older Patients with IPF.
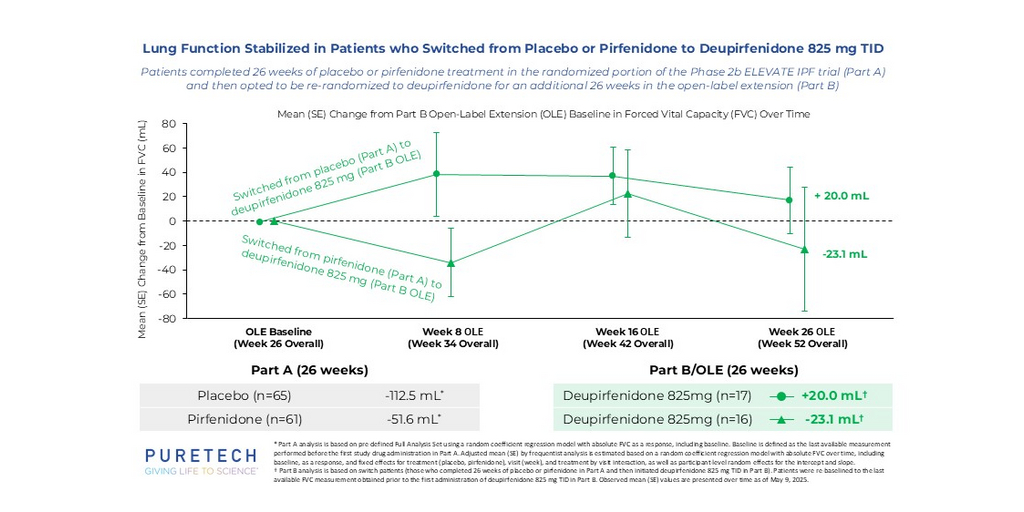
PureTech Presents New Data from Phase 2b Open-Label Extension Study of Deupirfenidone (LYT-100), Further Supporting Strong and Durable Efficacy and Potential to Serve as New Standard of Care in IPF
businesswire.com
2025-09-29 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Presents New Data from Deupirfenidone Phase 2b Open-Label Extension, Further Supporting Potential to Serve as New Standard of Care in IPF.

What Makes PureTech Health (PRTC) a New Buy Stock
zacks.com
2025-09-16 13:02:09PureTech Health (PRTC) has been upgraded to a Zacks Rank #2 (Buy), reflecting growing optimism about the company's earnings prospects. This might drive the stock higher in the near term.
No data to display

PureTech to Present at the Leerink Partners Global Healthcare Conference
businesswire.com
2026-02-25 02:00:00BOSTON--(BUSINESS WIRE)--PureTech to Present at the Leerink Partners Global Healthcare Conference.

PureTech Announces Orphan Drug Designations Granted by the U.S. Food and Drug Administration and European Commission for Deupirfenidone (LYT-100) in Idiopathic Pulmonary Fibrosis
businesswire.com
2026-02-19 12:36:00BOSTON--(BUSINESS WIRE)--PureTech Announces Orphan Drug Designations Granted by the U.S. Food and Drug Administration and European Commission for Deupirfenidone in IPF.

PureTech Health plc (PRTC) Presents at 44th Annual J.P. Morgan Healthcare Conference Transcript
seekingalpha.com
2026-01-14 23:35:58PureTech Health plc (PRTC) Presents at 44th Annual J.P. Morgan Healthcare Conference Transcript

PureTech to Present at 44th Annual J.P. Morgan Healthcare Conference
businesswire.com
2026-01-06 07:00:00BOSTON--(BUSINESS WIRE)--PureTech to Present at 44th Annual J.P. Morgan Healthcare Conference.

PureTech Appoints Robert Lyne as Chief Executive Officer
businesswire.com
2025-12-18 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) ("PureTech" or the "Company"), a hub-and-spoke biotherapeutics company dedicated to giving life to science and transforming innovation into value, today announces that the Board of Directors has appointed Robert Lyne as Chief Executive Officer (CEO), and as a member of the Board of Directors, effective immediately. Robert Lyne commented: “I'm honored to lead PureTech as CEO at such an important moment in its evolution. Over.

Puretech gains after FDA meeting post-Phase II trial
proactiveinvestors.co.uk
2025-12-08 06:25:14PureTech Health (LSE:PRTC, NASDAQ:PRTC, OTC:PTCHF) shares rose 4.9% to 128p on Wednesday after the company said it had completed a successful end-of-Phase 2 meeting with the US Food and Drug Administration for deupirfenidone, its lead idiopathic pulmonary fibrosis candidate. The regulator's feedback supports advancement into a pivotal Phase 3 trial and confirms the suitability of the streamlined 505(b)(2) regulatory pathway, reducing a major point of uncertainty around the programme.

Cavendish sees Poolbeg’s POLB 001 gaining strategic importance as CRS research expands
proactiveinvestors.com
2025-12-08 06:15:00Cavendish has taken an upbeat view of Poolbeg Pharma PLC's (AIM:POLB, OTC:POLBF) prospects after the company’s anti-inflammatory candidate POLB 001 was...

Greengage plans AQSE float with bold Bitcoin-backed lending strategy
proactiveinvestors.com
2025-12-08 06:10:00Fintech Greengage has set out plans to list on the Access segment of the Aquis Growth Market... and it is doing so with an unusually ambitious twist....

Puretech gains after FDA meeting post-Phase II trial
proactiveinvestors.com
2025-12-08 05:54:00PureTech Health (LSE:PRTC, NASDAQ:PRTC, OTC:PTCHF) shares rose 4.9% to 128p on Wednesday after the company said it had completed a successful end-of-Phase 2...

Coinsilium notes Greengage’s plan to float on Aquis
proactiveinvestors.com
2025-12-08 04:27:00Coinsilium Group Limited (AQSE:COIN, OTCQB:CINGF) has welcomed news that Greengage, one of its portfolio companies, intends to list on the Aquis Stock...

PureTech Announces Successful End-of-Phase 2 Meeting with FDA for Deupirfenidone (LYT-100) in Idiopathic Pulmonary Fibrosis
businesswire.com
2025-12-08 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Announces Successful End-of-Phase 2 Meeting with FDA for Deupirfenidone (LYT-100) in Idiopathic Pulmonary Fibrosis.

PureTech's Founded Entity Gallop Oncology Announces Positive Initial Topline Data from Phase 1b Trial of LYT-200 in Relapsed/Refractory Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome
businesswire.com
2025-12-05 13:00:00BOSTON--(BUSINESS WIRE)--PureTech's Founded Entity Gallop Oncology Announces Positive Initial Topline Data for LYT-200 in Relapsed/Refractory Acute Myeloid Leukemia.

PureTech Presents New Phase 2b Analyses Demonstrating Consistent Safety and Efficacy of Deupirfenidone in Older Patients with Idiopathic Pulmonary Fibrosis (IPF), a Historically Undertreated Group
businesswire.com
2025-10-22 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Presents New Phase 2b Analyses Demonstrating Consistent Safety and Efficacy of Deupirfenidone in Older Patients with IPF.

PureTech Presents New Data from Phase 2b Open-Label Extension Study of Deupirfenidone (LYT-100), Further Supporting Strong and Durable Efficacy and Potential to Serve as New Standard of Care in IPF
businesswire.com
2025-09-29 02:00:00BOSTON--(BUSINESS WIRE)--PureTech Presents New Data from Deupirfenidone Phase 2b Open-Label Extension, Further Supporting Potential to Serve as New Standard of Care in IPF.

What Makes PureTech Health (PRTC) a New Buy Stock
zacks.com
2025-09-16 13:02:09PureTech Health (PRTC) has been upgraded to a Zacks Rank #2 (Buy), reflecting growing optimism about the company's earnings prospects. This might drive the stock higher in the near term.